Notifications
11 minutes, 16 seconds
-369 Views 0 Comments 0 Likes 0 Reviews
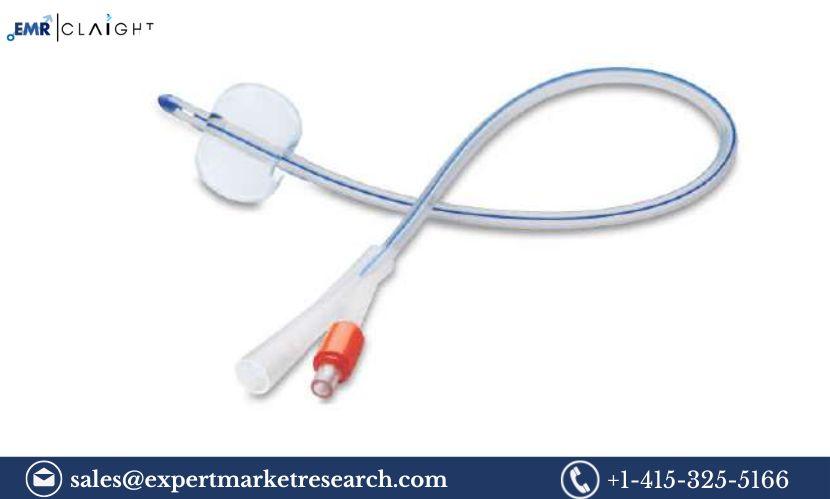
Foley catheters are essential medical devices used for the temporary drainage of urine from the bladder. These catheters are widely used in hospitals, clinics, and healthcare facilities, particularly for patients who are unable to urinate independently due to illness, injury, or surgery. The Foley Catheter Manufacturing Plant Project Report outlines the comprehensive steps required to establish a manufacturing unit for producing these vital medical devices. This report provides insights into the manufacturing process, market trends, required equipment, regulatory considerations, and financial aspects necessary for setting up a successful Foley catheter production facility.
The global medical device market is experiencing steady growth, driven by increasing healthcare demands, aging populations, and advancements in medical technologies. Foley catheters are a critical component of this market, especially in urology and intensive care units (ICUs). The rising incidence of chronic conditions, such as urinary retention, kidney diseases, and neurological disorders, is further contributing to the growing demand for catheters.
Get a Free Sample Report with Table of Contents@
The manufacturing of Foley catheters involves several stages to ensure that the final product is durable, safe, and comfortable for patients. Below is a detailed breakdown of the key steps involved:
The first step in the manufacturing process is sourcing high-quality materials. Foley catheters are typically made from medical-grade silicone, latex, or polyurethane, all of which must meet strict regulatory standards. Silicone is the most commonly used material due to its biocompatibility, flexibility, and resistance to infections.
The raw materials are fed into an extrusion machine where they are heated and molded into long tubes, which will form the body of the Foley catheter. This process ensures that the tubing has the required thickness, flexibility, and smoothness. The extrusion process can also include the addition of specific features, such as color coding, for easier identification of catheter sizes.
Foley catheters typically have an inflatable balloon at the tip to keep the catheter in place within the bladder. The balloon is created by molding a thin, flexible balloon material onto the catheter's distal end. The balloon is inflated using sterile water or saline after the catheter is inserted, which helps secure the device in position.
A one-way valve is installed into the balloon's inflation port to prevent the catheter from losing its inflated position after insertion. This valve is a crucial component of the catheter's functionality, ensuring it remains securely in place.
Sterilization is a critical step in the production of Foley catheters to ensure that the final product is free from bacteria and other contaminants. There are several sterilization methods, with ethylene oxide (EO) gas sterilization being the most common. This process ensures that the catheters meet the necessary safety standards for medical devices.
Once sterilized, the catheters are carefully packaged in sterile, tamper-evident packaging to maintain their sterility until they are used in a clinical setting. The packaging is designed to protect the catheter during storage and transport while providing clear information on the product’s specifications, such as size, material, and sterilization details.
Throughout the production process, quality control (QC) checks are essential to ensure that the catheters meet industry standards for durability, flexibility, safety, and sterility. QC procedures include testing for balloon inflation, tube strength, material integrity, and leak testing. In addition, the final product undergoes inspections to verify that the packaging is intact and properly sealed.
To establish a Foley Catheter Manufacturing Plant, it is crucial to invest in specialized equipment and infrastructure that can meet the stringent requirements of medical device manufacturing. Below are some of the essential components:
Given the medical nature of the product, a cleanroom environment is necessary for manufacturing, packaging, and sterilizing the catheters to prevent contamination.
The materials used in Foley catheters must pass biocompatibility tests to ensure that they do not cause adverse reactions when in contact with human tissues.
Setting up a Foley Catheter Manufacturing Plant requires significant capital investment. Some of the financial components to consider include:
The initial investment includes costs for land acquisition, facility construction, purchase of machinery and equipment, raw materials, and regulatory approvals. The setup may also include expenses for obtaining certifications and building the necessary infrastructure, such as cleanrooms and storage facilities.
Ongoing operational costs for the manufacturing plant include:
The profitability of a Foley catheter manufacturing plant is influenced by factors such as product demand, production efficiency, and the ability to maintain quality standards. The healthcare industry’s demand for reliable and high-quality catheters ensures a stable market, with opportunities for long-term profitability.
1. What materials are used to make Foley catheters? Foley catheters are typically made from silicone, latex, or polyurethane. Silicone is preferred for its biocompatibility and flexibility.
2. What equipment is required to manufacture Foley catheters? Key equipment includes extrusion machines, balloon molding machines, valve insertion machines, sterilization chambers, packaging machines, and quality control systems.
3. What regulatory certifications are needed for Foley catheter manufacturing? Manufacturers must comply with FDA regulations, obtain ISO 13485 certification, and meet CE marking requirements for European markets.
4. How long does it take to set up a Foley catheter manufacturing plant? Setting up a plant can take anywhere from 12 to 18 months, depending on the scale of the facility, obtaining regulatory approvals, and the complexity of the manufacturing process.
Media Contact:
Company Name: Claight Corporation
Contact Person: Lewis Fernandas, Corporate Sales Specialist — U.S.A.
Email: sales@expertmarketresearch.com
Toll Free Number: +1–415–325–5166 | +44–702–402–5790
Address: 30 North Gould Street, Sheridan, WY 82801, USA
Website: www.expertmarketresearch.com
Aus Site: https://www.expertmarketresearch.com.au
Foley Catheter Manufacturing Plant Project Report Foley Catheter Manufacturing Plant Project Foley Catheter Manufacturing Plant Setup

